What is a medical PCB
A medical PCB is a printed circuit board used in medical devices. Today we live in a time with the best medical tools ever. People recover from diseases that used to kill them. Patients recover from attacks on their bodies or from organ failure. Doctors are real-life heroes and they deserve praise. Our unnamed PCB heroes also deserve praise. Designers and makers of medical electronics carry a heavy duty every day. They make sure medical devices work. They save lives.

Medical PCB applications
Medical care covers many things. It covers small wearable tools that help health. It also covers big whole-body imaging systems that check organs. Patient care, research, and training of medical staff are all part of the medical PCB world. Now we see many types of PCBs from cardiovascular boards to imaging system boards. These PCBs are used in pacemakers, defibrillators, and heart monitors. They are also used in MRI, CT scan, and ultrasound machines. You also find PCBs in temperature monitors, blood glucose meters, and electronic muscle stimulators.
Key features of medical PCBs
1) Safety and reliability
Safety and reliability are the core needs for medical PCBs. Life-support products and some high-end products follow IPC-3 level standards. Non-life-support or mid-to-low-end products follow IPC-2. Big medical terminals often create their own company standards. These standards control product reliability. This point is very important.
2) Long service life
Most medical products have warranties of five years or more. Large medical devices often require at least ten years. These products must work for a long time. This need changes design and process from ordinary PCBs.
3) Wide range of levels
Medical products range from consumer medical items to high-reliability, high-stability mid-to-high-end products. They also include very small, highly integrated portable devices and smart wearable medical devices with many functions. Design and manufacturing must match this range.
4) Conservative product adoption
New medical tech and new products reach the market slowly. Validation tests for new products are repeated. Full evaluations are often required before a new product is accepted.
5) Traceability
Medical products need strict process records and traceability. Some large medical terminals require traceability of PCB processing records for up to ten years.
6) Different process and fire rating needs
Process steps and fire ratings for medical PCBs can differ from normal PCBs. For example, methods for HASL or cleaning may not be the same as for ordinary boards. When repair is needed, careful process control is required to keep quality and safety.
7) Low volume, high process demand
Medical PCBs usually have small order quantities. They need many special processes like back drilling, multiple laminations, mixed lamination, high precision, special impedance control, thick copper, heavy gold, and embedded copper blocks. Price is not the top concern for many medical PCBs. The focus is on quality and process capability. This makes them different from ordinary PCBs.
Among all medical PCB materials, fr4 is the most common. For medical PCB design, choice of fr4 grade depends on product position and material cost control. Large hospital groups must choose manufacturers that can prove reliability for medical PCBs.
What to pay attention to for medical-grade PCB
When you pick PCB materials and specs for your medical device, you face many choices. Choosing the right PCB specs gives you the best device performance. Below are the main PCB types used in medical devices and the reasons they are good choices.
Fleksibilna štampana pločica
Flexible PCBs are often the first choice in many medical devices. Medical devices are often placed where they need to bend or flex. The stretch and bend of a flexible PCB make it a good fit. Use flexible PCBs when your device will fold or wrap.
Flexible aluminum PCB
Flexible PCBs work for many medical electronics. But some high-power devices need stronger boards. Flexible aluminum PCBs are good for high-power apps because they move heat well. Main uses include scanning and surgical lighting.
PCB microcircuit
You may also consider flexible PCB microcircuits. Like flexible PCBs, they bend. But they let you use smaller boards with higher performance. Because of their design, microcircuit PCBs often go in small medical items like industrial sensors, wearables, and hearing aids.
Polyimide PCB material
When you choose a board substrate, polyimide is often a good pick. Polyimide boards have high tensile strength and good flexibility. These materials are very durable. They handle heat very well. They resist chemicals. They work for prosthetics and implantable devices.
Keramička štampana pločica
Ceramic PCBs are a great choice for devices that need low thermal expansion and high thermal conductivity. These boards can run at very high temperatures. They give great high-frequency performance. They allow dense traces and they resist chemical corrosion well.
Rigid-flex PCB
Rigid-flex boards mix the benefits of flexible circuits with the strength of rigid boards. Flexible layers sit between rigid layers that hold conductors. They have plated vias to connect layers. These boards are tougher than flex-only boards. They are lighter and thinner than rigid boards. They show more design freedom. You see them in consumer electronics, lighting, contract manufacturing, and instruments.
Traceability requirements for medical device PCBs
Manufacturers often use process audits done by external assessors or by the company itself to verify they meet industry rules and guidelines. Good records that meet medical traceability rules are essential for accurate assessment. In a highly regulated field, your contract manufacturer (CM) must be a loyal partner to help you meet this key quality management need.
What is traceability and why it matters
The ability to track your steps is very useful. It lets you review past practices with knowledge of the outcome. This hindsight helps only if the records include data or information that identifies actions or areas for improvement. For building circuit boards and electronic products, traceability can be defined like this:
Definition: Traceability in PCBA development means the ability to track and record the raw materials and components used during the whole manufacturing process by specific data (for example, product serial number). It also covers the equipment and processes used, including inspection and verification techniques.
When you think about why traceability matters for medical PCBA and electronics, three main attributes stand out.
1) Quality management
For medical OEMs and suppliers, ISO 13485 sets widely accepted requirements for almost all parties in the medical device lifecycle. Companies that supply tools and parts to medical device users must follow this standard, including its documentation and traceability rules. OEMs may ask that other links in the supply chain also follow this standard.

2) Process agility
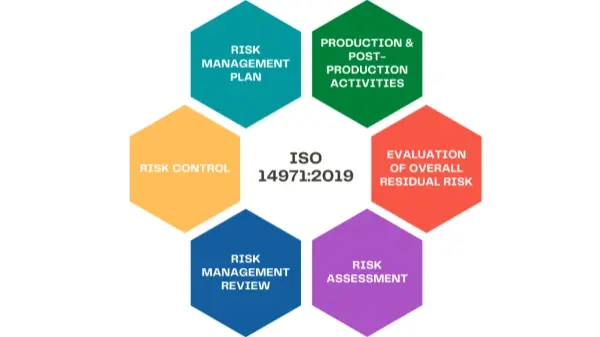
Traceability needs in medical device PCBA development let manufacturers make and run good risk management plans. ISO 14971 is also a recognized international standard. It gives a full risk management framework. It includes tools like Preliminary Hazard Analysis (PHA), Hazard and Operability study (HAZOP), and Failure Mode and Effects Analysis (FMEA). Using one of these methods is key to keep process agility. With process agility, you can make fast changes and raise the overall reliability of product development.
3) Legal protection
Meeting medical PCB traceability rules also gives legal protection if an incident happens. An incident may cause patient harm or other major damage. In that case, the ability to show you followed standards is very valuable.
How to make sure you meet traceability needs for medical PCBs
There are many benefits of tracking PCBA development across the supply chain. To do it well, all parties must set rules to make sure documents are collected and available.
Steps to meet traceability needs
Follow the right industry standards. Use medical electronics and device standards that apply to your product.
Create a traceability agreement like AABUS between users and suppliers. This agreement states the traceability needs and how suppliers will meet them.
Work only with certified contract manufacturers who hold important medical device certifications.
Keep clear process records. Track materials, batches, and inspection results. Link these records to product serial numbers or lot numbers.
Keep long-term records for products that need them. Some devices need ten years of process traceability.
Use tested risk management tools like PHA, HAZOP, and FMEA. Link these results to traceability records.
Make sure your CM can help with regulatory audits. They must show records quickly and clearly.
Summary of technical demands for medical PCBs
Medical PCBs need high quality in both design and process. They also need clear records and testing. The main differences from general PCBs are:
Higher safety and reliability needs.
Longer service life.
Range from simple consumer medical items to complex, high-reliability systems.
Slower adoption of new tech.
Strong traceability and long record keeping.
Special processes like back drilling, multiple lamination, mixed lamination, tight impedance, thick copper, thick gold, and embedded copper are common.
fr4 is common, but other materials like polyimide, ceramic, and rigid-flex are important for special needs.
Philifast — how we help medical device makers
Philifast makes and assembles medical PCBs and PCBAs. We know what the medical field needs. We work to meet strict rules on safety, reliability, and traceability. Below are key points about Philifast that match the needs above.
Our quality and certification
Philifast follows international quality and safety standards. We are UL certified and RoHS compliant. We also follow ISO and other international standards.
We keep full process records that help with traceability. We can keep ten-year records if your project needs them.
We work with customers to meet ISO 13485 quality management needs. We also help apply risk management tools like ISO 14971 methods.
Our process capability
We can do back drilling, multiple laminations, and mixed lamination.
We can meet high-precision needs and tight impedance control.
We can build boards with thick copper, heavy gold plating, and embedded copper blocks.
We can handle small batch, high-mix production with strict quality checks.
We use professional automation and controlled processes to keep quality high and lead times short.
Material and product range
We make fr4 boards, polyimide boards, ceramic boards, rigid-flex, flex, and flex-aluminum boards.
We build microcircuit PCBs for small devices and high-density boards for imaging equipment.
We support high-power boards for surgical and lighting systems.
Traceability and documentation
Philifast keeps full material and process trace records.
We link materials and components to serial numbers and lot codes.
We assist customers with AABUS traceability setup and supplier agreements.
We support audit needs and can provide records for regulatory review.
Why choose Philifast
We focus on medical-grade reliability. You get boards that meet strict rules.
We offer flexible production for small and large projects.
We help with documentation and audits. We act as a trusted CM partner.
We care about quality more than price when lives are at stake. We put safety first.
Final notes and call to action
Medical PCB work is serious. It needs deep process control, strong materials, and full traceability. If you design or make medical devices, pick a PCB partner that shares your view on safety and quality. Philifast offers technical know-how, certified quality, and full traceability. We can help with design guidance, process choices, and manufacturing. Contact Philifast to discuss your medical PCB needs. We can help you choose the right board type, set up traceability, and meet regulatory needs.
Često postavljana pitanja
Typical uses include diagnostic equipment, patient monitors, imaging systems, implantable devices (where applicable), infusion pumps, surgical tools and wearable medical sensors.
Manufacturers commonly work under medical device quality systems and reference standards such as ISO 13485, applicable IEC standards (device-specific), and IPC requirements; always confirm specific device certification needs with your OEM.
Choices depend on application: biocompatible or low-outgassing materials, high-reliability laminates, stable dielectric materials, and finishes compatible with sterilization or long-term use are commonly selected.
Assembly often uses stricter cleaning, low-residue fluxes, controlled soldering profiles, selective conformal coatings, and additional inspections to minimize contamination and ensure long-term reliability.
Critical—lot traceability for materials, process records, inspection reports, and serial-level documentation are usually required to meet regulatory and customer demands.
Yes—designers must consider redundancy for safety-critical functions, grounding and isolation for patient safety, controlled impedance for signal integrity, and manufacturability for reliable yields.

